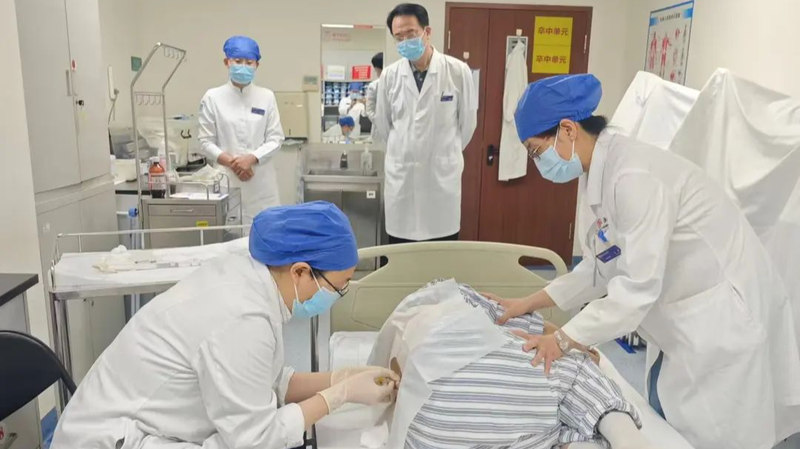
Biogen marked a milestone on Tuesday, commercially launching Tofersen in the Chinese mainland. The gene-targeted therapy is the first approved treatment for amyotrophic lateral sclerosis (ALS) caused by mutations in the SOD1 gene.
ALS progressively destroys motor neurons in the brain and spinal cord, leading to muscle weakness, atrophy and, without intervention, fatal respiratory failure within three to five years. For patients with the SOD1 variant, which often appears around age 50 and begins in the limbs, options have been limited – until now.
The first injection at Peking University Third Hospital signals the clinical availability of China's first therapy targeting SOD1-ALS. Tofersen is an antisense oligonucleotide (ASO) designed to reduce the synthesis of the toxic superoxide dismutase 1 protein, slowing motor neuron damage and disease progression.
This launch not only aligns the Chinese mainland with global biotech advancements but also offers tangible hope to patients, caregivers and healthcare systems grappling with a relentless disease. As research continues, stakeholders across medicine, business and policy will watch closely to see how Tofersen reshapes the ALS landscape.
Reference(s):
Breakthrough gene-targeted drug for ALS patients launched in China
cgtn.com